Identifiers and Description
Identifiers and Description
Gene Model Identifier
TTHERM_00411810Standard Name
CDK1 (Cyclin-Dependent Kinase 1)Aliases
PreTt21599 | 44.m00266 | 3714.m00112Description
CDK1 cyclin-dependent kinase-like Serine/Threonine kinase family protein; Cyclin-dependent protein kinase; localizes to domains associated with mature basal bodies of ciliary rows and to both the polykineties and haplokinety of the oral apparatus; partial knockout implicates this CDK in chromatin condensation; Cyclin-dependent kinaseGenome Browser (Macronucleus)
Genome Browser (Micronucleus)
 External Links
External Links
 Gene Ontology Annotations
Gene Ontology Annotations
Cellular Component
- cell cortex (IDA) | GO:0005938
Molecular Function
- cyclin binding (ISS) | GO:0030332
- cyclin-dependent protein serine/threonine kinase activity (ISS) | GO:0004693
Biological Process
- cell cycle (IEP) | GO:0007049
- cortical cytoskeleton organization (IMP) | GO:0030865
- obsolete histone phosphorylation (IDA) | GO:0016572
 Domains
Domains
 Gene Expression Profile
Gene Expression Profile
 Vegetative Cell Cycle (Zhang et al.,
2023)
Vegetative Cell Cycle (Zhang et al.,
2023)
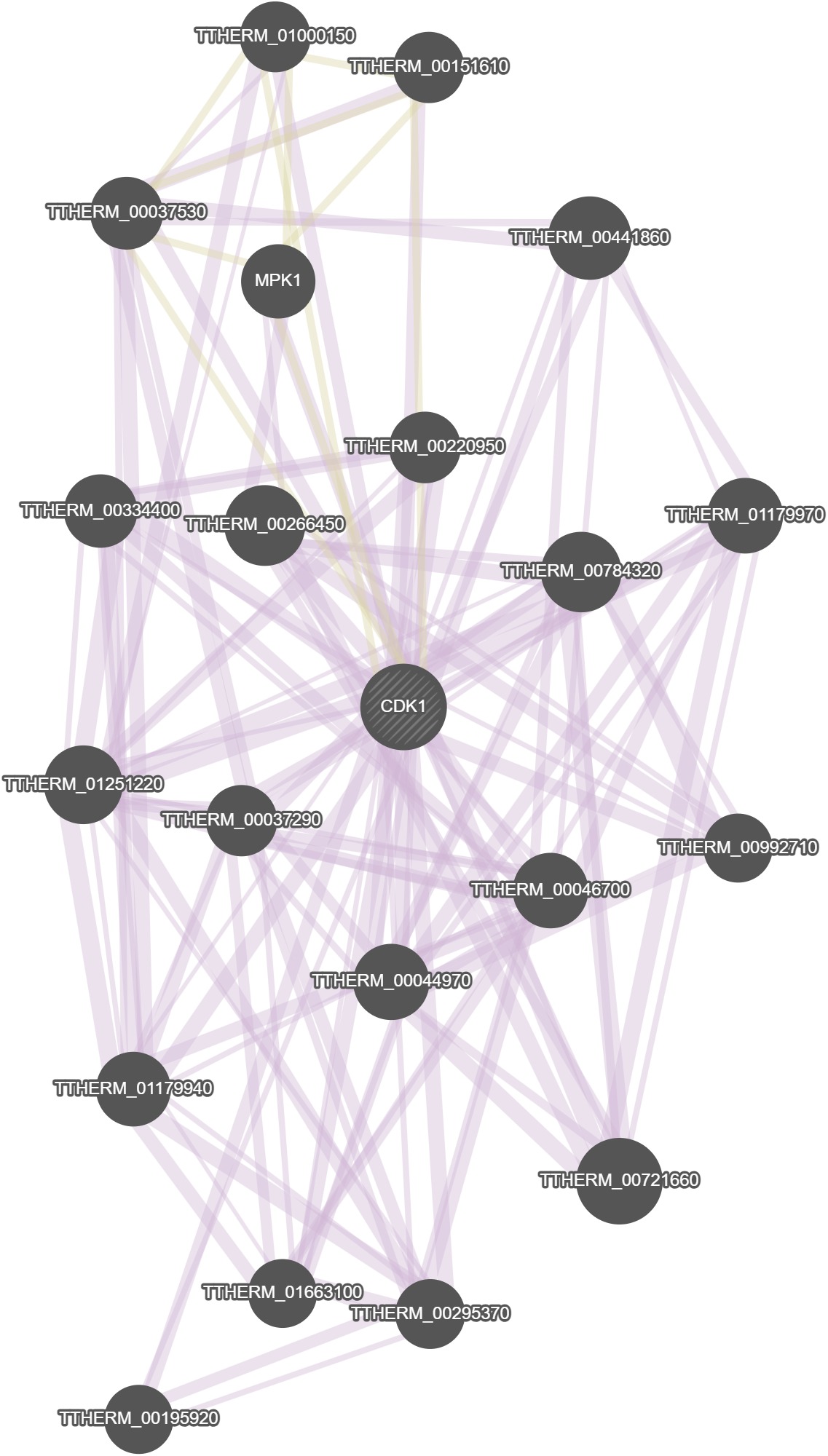
 GeneMania
GeneMania
 Tetrahymena Stock Center
Tetrahymena Stock Center
No Data fetched for Tetrahymena Stock Center
 Homologs
Homologs
No Data fetched for Homologs
 General Information
General Information
No. Gene Name(s) Paragraph Text 2212 CDK1 The level of Cdk1p fluctuates over the vegetative cell cycle, correlating with its histone H1 kinase activity. Cdk1p is associated with the basal bodies of the ciliary rows of the cell cortex and the oral apparatus. This localization, along with the phenotype of a partial CDK1 knockout phenotype of bent and buckled ciliary rows, suggests that Cdk1p is involved in cortical morphogenesis. 2211 CDK1 Tetrahymena thermophila Cdk1p shares homology with cdk homologs from other eukaryotes. It contains 11 catalytic domains characteristic of protein kinases, conserves all of the regulatory phosphorylation sites found in cdks, and has a slightly modified cyclin-binding PSTAIRE motif that is a hallmark of cdks. The Tetrahymena thermophila Cdk1p was also found to bind Saccharomyces cerevisiae p13suc1, a yeast cyclin. 2210 CDK1 Cyclin-dependent kinases (cdks) are a family of serine-threonine kinases that are involved in cell cycle control and cell division in eukaryotes. Cdks are catalytic subunits that are activated by association with proteins called cyclins, forming cyclin-cdk complexes. Cdk kinase activity is regulated by cyclin binding, phosphorylation and dephosphorylation, protein degradation, protein-protein interactions with cdk inhibitors, and subcellular localization.
 Associated Literature
Associated Literature
- Ref:16933976: Eisen JA, Coyne RS, Wu M, Wu D, Thiagarajan M, Wortman JR, Badger JH, Ren Q, Amedeo P, Jones KM, Tallon LJ, Delcher AL, Salzberg SL, Silva JC, Haas BJ, Majoros WH, Farzad M, Carlton JM, Smith RK, Garg J, Pearlman RE, Karrer KM, Sun L, Manning G, Elde NC, Turkewitz AP, Asai DJ, Wilkes DE, Wang Y, Cai H, Collins K, Stewart BA, Lee SR, Wilamowska K, Weinberg Z, Ruzzo WL, Wloga D, Gaertig J, Frankel J, Tsao CC, Gorovsky MA, Keeling PJ, Waller RF, Patron NJ, Cherry JM, Stover NA,
- Ref:12183062: Zhang H, Huang X, Tang L, Zhang QJ, Frankel J, Berger JD (2002) A cyclin-dependent protein kinase homologue associated with the basal body domains in the ciliate Tetrahymena thermophila. Biochimica et biophysica acta 1591(1-3):119-128
- Ref:12045216: Doree M and Hunt T (2002) From Cdc2 to Cdk1: when did the cell cycle kinase join its cyclin partner? J Cell Sci 115(Pt 12):2461-4
- Ref:10329641: Mizzen CA, Dou Y, Liu Y, Cook RG, Gorovsky MA, Allis CD (1999) Identification and mutation of phosphorylation sites in a linker histone. Phosphorylation of macronuclear H1 is not essential for viability in tetrahymena. The Journal of biological chemistry 274(21):14533-6
- Ref:9442875: Morgan DO (1997) Cyclin-dependent kinases: engines, clocks, and microprocessors. Annu Rev Cell Dev Biol 13():261-91
- Ref:8995382: Sweet MT, Carlson G, Cook RG, Nelson D, Allis CD (1997) Phosphorylation of linker histones by a protein kinase A-like activity in mitotic nuclei. The Journal of biological chemistry 272(2):916-23
- Ref:7958616: Doree M and Galas S (1994) The cyclin-dependent protein kinases and the control of cell division. FASEB J 8(14):1114-21
- Ref:2065655: Roth SY, Collini MP, Draetta G, Beach D, Allis CD (1991) A cdc2-like kinase phosphorylates histone H1 in the amitotic macronucleus of Tetrahymena. The EMBO journal 10(8):2069-75
 Sequences
Sequences
>TTHERM_00411810(coding)
ATGAACAATAATACATTTGGCTCAGACAGATATGAAAAGCTACTTAAAGTAGGTGAAGGA
ACATATGGTGAAGTTTACAAGGCTAAAGACATTCAAAGCTCTGAAATTGTTGCTTTGAAG
AAGATTAAATTAGAAAATGAAGATGAAGGTGTCCCTTCAACTGCTCTAAGAGAAATTTCA
ATACTTAAAGAGTTACAACCACATCCCAACATCGTTTGCATGCATGAAGTTATTTATCAA
CCCTAAGAGAAGAAGTTATACCTTGTATTTGAATTTGTTGACTAAGACCTTAAAAAATTC
TTAGACTAATACCGCAAAGATAAAAAACTTTAACTTCGTCCTTACCAAATCAAACTCATG
ATGTATCAAATTTTGAATGGATTGAATTTCTGCCACAGCCGTAGAATTATTCATAGAGAT
TTAAAACCCTAAAACATTTTAATTGATGCTAAGGGAAATATTAAGATTGCTGATTTTGGT
TTGGCTAGAGCATTTGGTGTTCCTATCAAGACTCTTACTCATGAAGTTGAAACTCTTTGG
TACAGAGCTCCCGAAATTCTTTTAGGCTAAAAGGCTTATAGTCTCGGAGTTGATATTTGG
AGTTTGGGTTGCATCTTCCACGAAATGGTTGAAAAGAGAGCTCTCTTCATGGGTGACTCT
GAAATTGATTAAATATTTAAAATATTCTAATACCATGGTACTCCTACTGAGTAGACATGG
CCTGCTTTGAAGGAATGTCCTTACTTTAAACCTATATATCCTCGTTTCAAAACTGCTGAT
CCTAAGACTTACTTTAAGAATTTCTGCGATAAAGGATTTGACCTTATTTAATAGATGATT
GCTCTTGACCCTGCTAAAAGAATATCAGTAAAGGATGCTCTCAGACATCCCTACTTTGAA
GATCTTAGCAGAGAAGATATAGCTAAATTTGAGCCTAATTAAGTTCATATGTACTGA>TTHERM_00411810(gene)
CACACAAAAAAACAAAAAACAAATAATTAATTATAAAGCAAAACAAAAATTTAAATTTAA
AGATAAAAGAGTATCAACTCTCATAAGAAAAATTATTGCTATCAAATATTCGAATTTAAG
CAAATAAATCAATCAATCAGTCAGTCATACATACATAATAGGATGAACAATAATACATTT
GGCTCAGACAGATATGAAAAGCTACTTAAAGTAGGTGAAGGAACATATGGTGAAGTTTAC
AAGGCTAAAGACATTCAAAGCTCTGAAATTGTTGCTTTGAAGAAGATTAAATTAGAAAAT
GAAGATGAAGGTGTCCCTTCAACTGCTCTAAGAGAAATTTCAATACTTAAAGAGTTACAA
CCACATCCCAACATCGTTTGCATGCATGAAGTTATTTATCAACCCTAAGAGAAGAAGTTA
TACCTTGTATTTGAATTTGTTGACTAAGACCTTAAAAAATTCTTAGACTAATACCGCAAA
GATAAAAAACTTTAACTTCGTCCTTACCAAATCAAACTCATGATGTATCAAATTTTGAAT
GGATTGAATTTCTGCCACAGCCGTAGAATTATTCATAGAGATTTAAAACCCTAAAACATT
TTAATTGATGCTAAGGGAAATATTAAGATTGCTGATTTTGGTTTGGCTAGAGCATTTGGT
GTTCCTATCAAGACTCTTACTCATGAAGTTGAAACTCTTTGGTACAGAGCTCCCGAAATT
CTTTTAGGCTAAAAGGCTTATAGTCTCGGAGTTGATATTTGGAGTTTGGGTTGCATCTTC
CACGAAATGGTTGAAAAGAGAGCTCTCTTCATGGGTGACTCTGAAATTGATTAAATATTT
AAAATATTCTAATACCATGGTACTCCTACTGAGTAGACATGGCCTGCTTTGAAGGAATGT
CCTTACTTTAAACCTATATATCCTCGTTTCAAAACTGCTGATCCTAAGACTTACTTTAAG
AATTTCTGCGATAAAGGATTTGACCTTATTTAATAGATGATTGCTCTTGACCCTGCTAAA
AGAATATCAGTAAAGGATGCTCTCAGACATCCCTACTTTGAAGATCTTAGCAGAGAAGAT
ATAGCTAAATTTGAGCCTAATTAAGTTCATATGTACTGAATATGAAAACAAACAACAACT
AATTAATTAATTAACAAACAAATTAATTGCAAATAAATTTTTATTTAAGAATTGATAATG
AATGAATGAAACAACCATATTATTAATTCAAAAATATAATAATTAATATCCGAATCAAAA
ATAAATAAATAAAAAAATAAATACCTACATATACAGAATCTCATAAAAATAATTTACTCA
TCTATTCTTATATTTACTCCCATCATAACCAATATATTTTTTAGCTTAAATTGTTAATGA
ATTCTTTTTGGGTCTATATATATATATTATAAAATATTGCAAATATTGTAATATATTTTA
TATTT>TTHERM_00411810(protein)
MNNNTFGSDRYEKLLKVGEGTYGEVYKAKDIQSSEIVALKKIKLENEDEGVPSTALREIS
ILKELQPHPNIVCMHEVIYQPQEKKLYLVFEFVDQDLKKFLDQYRKDKKLQLRPYQIKLM
MYQILNGLNFCHSRRIIHRDLKPQNILIDAKGNIKIADFGLARAFGVPIKTLTHEVETLW
YRAPEILLGQKAYSLGVDIWSLGCIFHEMVEKRALFMGDSEIDQIFKIFQYHGTPTEQTW
PALKECPYFKPIYPRFKTADPKTYFKNFCDKGFDLIQQMIALDPAKRISVKDALRHPYFE
DLSREDIAKFEPNQVHMY